 STANDARD Q COVID Flu Ag Combo
STANDARD Q COVID Flu Ag Combo Moscow
from 4 pcs
573.26
$/pcsTo buy wholesale BIOCREDIT CoviFlu Ag Duo covid test, Type A and Type B flu test from IP Yafarov Ramil Ziatovich, contact the supplier via the messenger, request a callback or call the phone number.
 STANDARD Q COVID Flu Ag Combo
STANDARD Q COVID Flu Ag Combo Moscow
from 4 pcs
573.26
$/pcs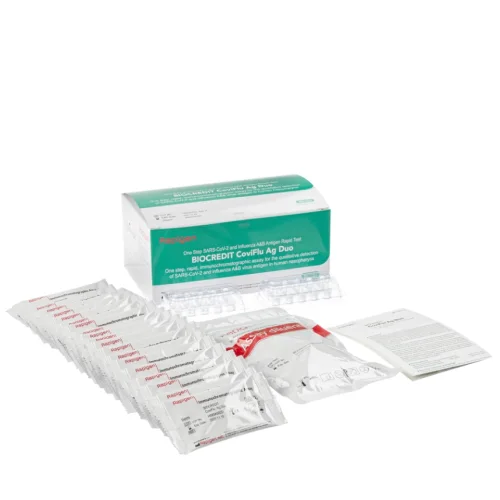
Moscow
from 1 pcs
226.76
$/pcs
Moscow
from 1 pcs
114.52
$/pcs
Moscow
from 5 pcs
191.09
$/pcs
Moscow
from 10 pcs
414.02
$/pcs STANDARD Q COVID-19 Ag Home Test, 1 pc.
STANDARD Q COVID-19 Ag Home Test, 1 pc.Moscow
from 100 pcs
21.02
$/pcsA set of reagents for immunochromatographic detection of SARS-CoV-2 antigen and influenza virus type A and type B antigens in a smear from the human nasopharynx (BIOCREDIT CoviFlu Ag Duo) is designed to detect antigen specific to the SARS-CoV-2 coronavirus in smears taken from the nasopharynx. A test kit of reagents for qualitative determination of the presence of SARS-CoV-2 antigen and influenza virus type A and type B antigens in a nasopharyngeal smear in persons with clinical symptoms of respiratory disease with suspected COVID-19 infection or acute respiratory viral disease. Using a probe, take a smear from the nose, immerse it in a tube with a medium, perform circular movements, squeeze the probe against the wall, close the tube with a dropper nozzle and then apply 3-4 drops of the medium to the sample window on the cassette, where there is a membrane strip with antibodies specific to viral antigens. During chromatography, colored transverse lines are formed on the membrane: one control line if there is no virus and the test is in working condition, control and test lines if the virus is present in the sample. The BIOCREDIT CoviFlu Ag Duo kit includes two cassettes: one for detecting the SARS-CoV-2 coronavirus antigen (nucleocapsid protein N), the other for detecting influenza A and B antigens, respectively. On the second there are sections for the control line and two test lines. Thus, testing of patients with clinical symptoms of acute respiratory viral disease will confirm or exclude three dangerous acute respiratory viral infections at once: COVID-19, influenza A and influenza B. Clinical trials in the territory of the Russian Federation have shown the absence of cross-reactivity with other viral and bacterial respiratory pathogens, which could lead to false positive results. Composition: Version 1. 1. Test cassette (in individual foil packaging complete with desiccant) - 20 pcs. 2. A test tube with a buffer for extracting a biological sample - 20 pcs. 3. Nozzle with dropper - 20 pcs. 4. Medical disposable sterile probe according to TU 32.50.13-002-28731857-2020 RU No. RZN 2021/13989 - 20 pcs. 5. Test tube stand - 2 pcs. 6. Instructions for the medical device - 1 pc. Version 2. 1. Test cassette (in individual foil packaging complete with desiccant) - 20 pcs. 2. A test tube with a buffer for extracting a biological sample - 20 pcs. 3. Nozzle with dropper - 20 pcs. 4. Test tube stand - 2 pcs. 5. Instructions for the medical device - 1 pc. Diagnostic specificity: 100%. Sensitivity: 100%. Execution time: 10-15 minutes. Registration certificate: RU No. RZN 2022/16435 dated January 27, 2022. Lot No. H080A002D Sub: A.
Manufacturer's article number
G68RHA20
View of goods
Covid + influenza A/B
Quantum Supplies
Brand / TM.
RapiGEN
Manufacturer country
South Korea
Barcode
8809408862072